You ready to have some fun? If you love color and rainbows, and don't mind a little experimenting, get ready to learn stuffs.
Let's start with a quick quiz. Get out a piece of paper, or open up a notepad app, and write down the color you see in this pretty flower. Blue? Purple? Some other color?
Okay, and again, look at the swatch of color below, and answer the same question. What color do you see?
We'll come back to the answers you've written after you watch this quick Minute Physics video, which claims that purple is not in the rainbow and that Newton's "violet" was really a dark blue.
Don't worry -- it's only a couple minutes long, and it'll be fun tearing it apart afterward.
After having watched the video, what do you think? Is Henry right ... is there no purple in the rainbow? Can I, a non-physicist, even hope to prove this guy wrong? Especially when so many reputable science websites have been referring to and using this video? Check out this article from the Smithsonian.
The nice thing about science is that anyone, anywhere, can test any scientific claim, provided they have enough tools to do the job. So, over the past month, I've been testing, interviewing, researching, and compiling, and now you can see all the gory results.
First off, let's talk about where Henry Reich (the Minute Physics guy) is right. By definition, violet is indeed the color at the end of the spectrum as we see it. It is monochromatic, meaning that its color consists of just one wavelength of color.
Also by definition, purple is indeed the combination of two colors: red and blue. In other words, it's bichromatic, having two wavelengths of color mixed together. So far it's not looking good for me -- Henry is winning.
And what about this picture I took of a rainbow that clearly shows purple on the bottom? Is this a win for me?
Alas, if you zoom in to the purple region and look carefully, you'll see the supernumerary rainbows Henry describes in the bottom right-hand corner, starting in the middle of the purple band. Are the additional reds at the bottom mixing with blue to make purple?
Dang! Looks like another point for Henry. Should I just give up now?
Not so fast, Dr. Eggman!
Henry is wrong on one very important detail. Violet is not a dark blue as he describes, and I can prove it.
To clarify what I mean, let's define "blueish" as describing a color that appears to our brains to contain only blue and no red; and "purplish" describes a color that appears to consist of both blue and red.
For example, check out this screen cap from Henry's video. Looking at the end of this rainbow, I see no purplish hues. There isn't even a hint of red in that violet end. Do you see any purple?
But I'm not done yet ... wait for it. When it comes to flowers, have you ever seen a blue violet? Or ever wondered why the poem says "violets are blue"? (The truth is they couldn't find a good rhyme for "purple.")
I will explain later two phenomenon as to why the picture above is missing the purplish hue at the end. Then you'll see how it's a terrible representation of the full spectrum as our brains see it.
Try this really fast ... do an image search on "real prism rainbow," and you will see about half with a blueish hue at the end, and half with a purplish hue.
Here's one pic I found that sports a purplish hue:
What's going on here? Why the contradicting pictures?
The best way to understand is to conduct your own experiments. The first thing I did when I heard the terrible news about poor purple was to find a prism-generated rainbow and check it out, myself.
I found one at work projected on a white table. I invited three victims to come and look at it with me, and asked, "What color do you see at the end of this spectrum?" They all answered: purple. (I took this picture with my iPhone 8.)
What color do you see at the end?
I also found a prism-generated rainbow at home. Check this out. I'll even zoom in for you in the image on the right. Also taken by my iPhone 8.
What color do you see at the end? Would you call it dark blue, or some kind of purple?
Note that any purplish hue you may see here is not coming from any supernumerary rainbows, which only exist in rainbows produced by thousands of rain droplets. The science here is different. Most spectrums created by prisms are "pure" ... you only get one iteration of the spectrum, and no overlaps.
Remember those colors I had you write down at the very beginning? Check your answers. The first picture is a violet I've owned over the past few years. It has always looked purplish to me. And the swatch of color comes from my prism picture just above -- again, it looks purplish to my eyes, not blueish. There's definitely more blue than red, but there does appear to be some red included.
In fact most people describe spectral violet as a mixture of 1 part red and 2 parts blue -- kind of like mixing blue and purple together.
Let me hit you with four color swatches and let you compare them all. The first is magenta -- 2 parts red and 2 parts blue -- definitely not in the rainbow. The second is a generated violet -- 1 part red and 2 parts blue -- which many seem to think appears in the rainbow. The third is the violet color grab (from the very top) -- it was in my rainbow, and the fourth is the blue demonstrated by Henry at around 1:26 (which never looks purple to me in his video -- BTW).
Which one of these looks blue to you (containing no red at all)? If you answered, "Only the last one," then perhaps Henry is wrong? And so are all the other reputable science sites that cited him?
Then again, I can understand the confusion -- red and blue are on opposite sides of the rainbow and every other color seems to make sense. Yellow falls between red and green. Orange is between red and yellow. Cyan falls between blue and green, and green between yellow and blue. But how does red wrap around all the way to the other end of the spectrum to make purple/violet?
Let's dive into this.
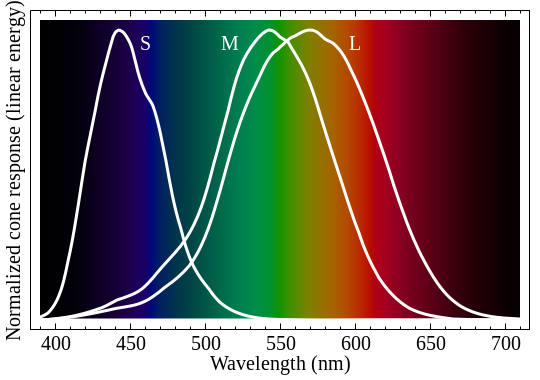
Most of us have three types of cones at the back of our eyes. One is mostly sensitive to red light, one to green, and one to blue. Wikipedia and several reputable websites show graphs similar to this to show each cone's sensitivity to light.
TV's and computer monitors take advantage of our cones. They're made of only three colors: red, green, and blue -- matching each of our three cones. And most all the colors we see can be emulated by different combinations of brightnesses. Here is one of many websites that explains this phenomenon. And here's a close-up of a typical monitor:
Consider pure yellow light, which activates both our red and green cones. A computer screen cannot shine yellow light, but it can shine red and green at the same time to emulate yellow. And our eyes can't tell the difference!
To experience this cool phenomenon, you can play around with this cute tool I found: an RGB Slider. If you go to the link, you can slide R(ed), G(reen), and B(lue) sliders to try to get any of 16 million colors.
Try these combinations:
Red = All R, No G, No B.
Yellow = All R, All G, No B.
Orange = All R, Half G, No B.
Cyan = No R, All G, All B.
Magenta = All R, No G, All B.
Purple = Half R, No G, Half B.
Violet = Half R, No G, All B.
White = All R, All G, All B.
Black = No R, No G, No B.
See what other colors you can produce. It all comes from just three colors with different levels of brightness. And it only works for human eyes. Animals looking at our screens would see something completely different and strange.
If it wasn't clear before, it should be clear now ... you can't have purplish hues without a hint of red. I challenge you ... dial Red down to zero, and then shifting only the Green and Blue sliders, can you get a purplish hue to appear?
Then, how in the world can we see purplish hues in the violet range (400-450nm)? I've come across a few theories among the Internets.
One theory is that the absence of green triggers our brains to see purplish hues. Maybe this is the case, but it doesn't satisfy me. When I hit the RGB slider with R=0, B=127, G=255, and then slowly lower green, I don't think I ever see purple as green disappears.
Another theory is that there's actually a second peak in the red cone sensitivity. This website shows the following picture (the extra gray line shows sensitivity of our rods for night vision). Note the small bump in the red sensitivity in the 380-430nm range -- which would explain how we see purplish hues. I like this theory, but unfortunately I can't find any credible sources to support this idea. And if it were true, why don't all websites show this bump?
Another theory is a combination of the two above, where if you look at the sensitivities in absolute values and not normalized to 100%, it could be that the red cones drop off in sensitivity slower than green -- so that when green drops out, there's enough residual red to give us purplish hues. Kind of like this ...
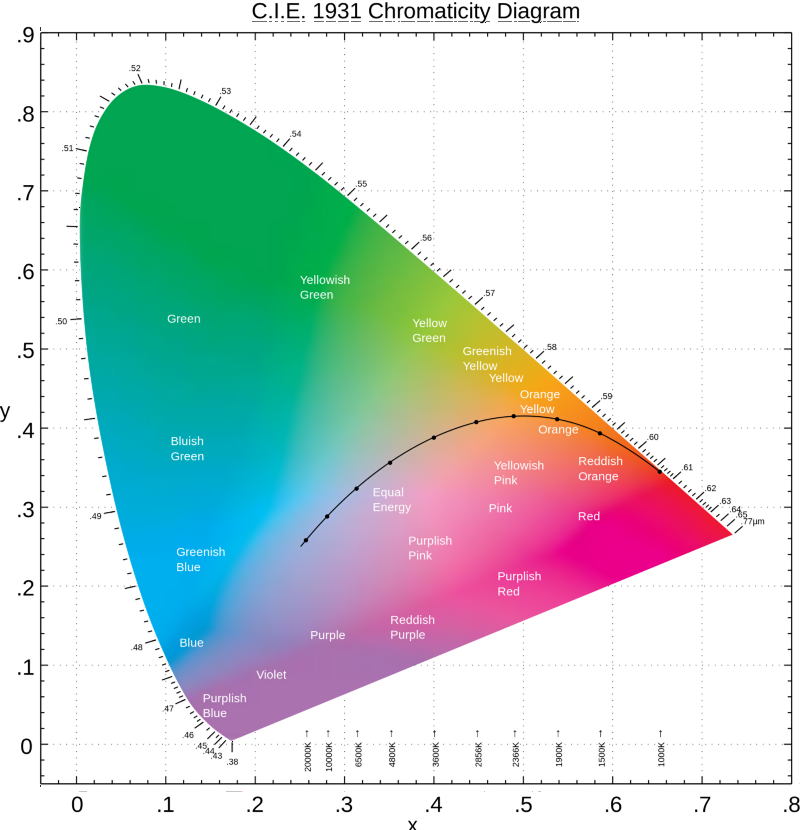
Whatever the cause, most humans seem to report purplish hues in the violet range, as evident in the CIE 1931 color matching scheme. This website goes to great lengths to describe all the experimentation with lights and all the formulae that went into being able to imitate practically all colors from just three lights: red, blue, green.
The CIE 1931 scheme plots most colors like so:
The pure spectrum exists along the upper outer edge, starting from the 770nm mark in the bottom right-hand red corner going along the green top around the 520nm mark, and then down the left side to the 380nm mark in the lower left-hand corner described as "Purplish Blue." The diagonal line along the bottom is not part of the spectrum. According to this scheme, "purple" is not in the rainbow, but "blueish purple" is. In other words, violet is a purplish hue, and not dark blue.
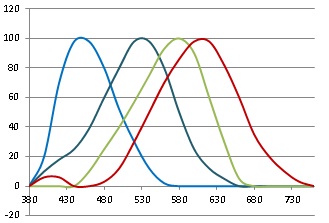
And check out from that CIE 1931 website, the standard RGB sensitivities used to emulate the spectrum ...
See that red bump in the 400-500nm range? I've scoured the webs, and I see that different versions of this chart are universally accepted anywhere it talks about CIE 1391 color matching. And all charts show two red bumps -- one big and one small.
Here's a nifty tool. It's a spectrum emulator, where you can shift the slider and see the resulting RGB makeup. Note how it shows purplish hues in the violet range (380-430). You can even see the "R" number rising in that range. Play around with the tool and have some fun with it.
If you made it this far, either you really like learning stuffs, or you still need more convincing. Why is it that Henry's picture of the spectrum up top shows deep blue and no purple at all? I believe this is due to two phenomenon.
#1) Our eyes have a hard time seeing violet. Once you get below 450nm, it becomes harder for our eyes to see it. In fact, in my many experimentations, there were plenty of times when I saw only the blue and no purplish hues. But if the light source is bright enough, violet becomes easier to see. It also appears to me not only to be purplish, but also "glowy" or "radioactive." That is, it seems to shimmer more than the other colors. In your experimentations, if you don't see "purple," you might want to try a stronger light source.
And #2) It turns out that some cameras have a hard time resolving violet as "purplish", but rather renders them as a dark blue color. Evidently the Panasonic FZ200 has this issue. If you're interested to learn more, here's a very informative video explaining these shortcomings in some cameras.
If such deficient cameras were to take pictures of spectra, they would only see blue at the end. I would further venture to postulate that if a human doesn't have a secondary peak in their red cones, they may likewise see just dark blue in the violet range. I found nothing supporting this, but could explain why some people claim not to see purplish hues in the violet range. To them, violet would look blue to them in real life and purple on a computer screen. Evidently, my iPhone 8 has no problem resolving violet as a purplish hue.
If you're still not convinced, you can check out these videos featuring "purple" lasers that shine a monochromatic 405nm -- well into the violet range. In each video, the lasers appear to be purple. You only need to watch a few seconds of each to get the idea. Keep in mind that lasers only emit one wavelength of light -- and not a mixture of red and blue.
And finally, let's come full circle by returning to rainbows. If I'm right, then shouldn't we be able to see the pure "purplish" violet in a rainbow? Turns out we can ... if the light source is bright enough and conditions are right. In fact, here's a screen shot from Henry's video, where it demonstrates a simulation of supernumerary rainbows based on raindrop sizes. At the very beginning of the clip, there are no supernumerary rainbows, and then as the drops get smaller, they appear and become more numerous.
I've found the actual website here if you want to play around with drop size. From that same website, this page shows the pure colors that combine to create the supernumerary images, and note that it depicts violet (400nm) as a purplish hue -- not dark blue.
And take a closer look at what color is depicted in this near-none-supernumerary rainbow simulation.
Doesn't that look purplish to you?
Now it's your turn. Do your own experiments. Find prism-generated rainbows (can't rely on rain-generated rainbows because of the supernumeraries) -- if you're observant enough, they're not too hard to find. And report back ... what color do you see at the end of your rainbows?
Update: 5-11-2022
As if we need further evidence, we now have "purple" lights that are peppering our nighttime cityscape. These were originally white LED street lights, but because of some defect, they have turned partially purple. You may see these if you live in North Carolina, Iowa, Florida, Arkansas, Texas, or Kentucky.
At first, I thought that the lights were losing the green, leaving red and blue to make purple. But then I wondered why my glasses weren't separating out the red, as I see with purple Halloween lights.
I decided to do a spectral analysis with my handy-dandy diffraction grating, and sure enough -- there is a big bump in the blue section, and no bump in the red. The violet light was too dim for me to make out, but the blue bump extended as far out as I could see. Either way it was clear -- this was NOT red+blue = purple.
So -- wow! These lights are really blue and violet. As you can see, my camera is picking up both blue and violet (depending, I think, on the angle).
Additionally, you may wonder why some of these lights look more "purple" than others. It turns out that it's not one LED light, but rather an array of many. The particular light pictured above had probably around 20 individual LEDs, a few of which were still shining white. So, one can watch a particular street light slowly transition from white to full "purple" as the LEDs go bad, one by one.
Enjoy these violet lights as long as you can, as they're slowly being replaced, and then we'll only have red+blue = purple lights left during Halloween and Christmas.